Capillary
| Capillary | |
|---|---|
| Transmission electron microscope image of a capillary with a red blood cell feces within the pancreas. The capillary lining consists of long, thin endothelial cells, connected by tight junctions. | |
| Blood flows away from the heart to arteries, which follow into arterioles, and then narrow further into capillaries. After the tissue has been perfused, capillaries branch and widen to become venules and then widen more and connect to become veins, which return blood to the heart. | |
| Code | TH H3.09.02.0.02001 |
Capillaries ( /ˈkæpɨlɛri/) are the smallest of a body's blood vessels and are parts of the microcirculation. They are only 1 cell thick. These microvessels, measuring 5-10 μm in diameter, connect arterioles and venules, and enable the exchange of water, oxygen, carbon dioxide, and many other nutrients and waste chemical substances between blood and surrounding tissues.[1] During embryological development, new capillaries are formed by vasculogenesis, the process of blood vessel formation occurring by a de novo production of endothelial cells and their formation into vascular tubes.[2] The term angiogenesis denotes the formation of new capillaries from pre-existing blood vessels.[3]
Contents |
Anatomy
Blood flows away from the heart via arteries, which branch and narrow into the arterioles, and then branch further still into the capillaries. After the tissue has been perfused, capillaries join and widen to become venules and then widen more to become veins, which return blood to the heart.
Capillaries do not function on their own. The "capillary bed" is an interweaving network of capillaries supplying an organ. The more metabolically active the cells, the more capillaries they will require to supply nutrients and carry away waste products.
A capillary bed can consist of two types of vessels: true capillaries which branch mainly from metarterioles and provide exchange between cells and the circulation. Secondly, capillary beds also consist of a vascular shunt which is a short vessel that directly connects the arteriole and venule at opposite ends of the bed.
Metarterioles provide direct communication between arterioles and venules and are important in bypassing the bloodflow through the capillaries. The internal diameter of 8 μm forces the red blood cells to partially fold into bullet-like shapes and to go into single file in order for them to pass through.
Precapillary sphincters are rings of smooth muscles at the origin of true capillaries that regulate blood flow into true capillaries and thus control blood flow through a tissue.
Types
There are three main types of capillaries:
- Continuous - They are continuous in the sense that the endothelial cells provide an uninterrupted lining, and only allow small molecules, like water and ions to diffuse through tight junctions which leave gaps of unjoined membrane which are called intercellular clefts. Tight junctions can be further divided into two subtypes:
-
- Those with numerous transport vesicles that are primarily found in skeletal muscles, finger, gonads, and skin.
- Those with few vesicles that are primarily found in the central nervous system. These capillaries are a constituent of the blood-brain-barrier.
- Fenestrated - Fenestrated capillaries (derived from "fenestra," Latin for "window") have pores in the endothelial cells (60-80 nm in diameter) that are spanned by a diaphragm of radially oriented fibrils and allow small molecules and limited amounts of protein to diffuse.[4][5] In the renal glomerulus there are cells with no diaphragms called podocyte foot processes or "pedicels," which have slit pores with an analogous function to the diaphragm of the capillaries. Both of these types of blood vessels have continuous basal lamina and are primarily located in the endocrine glands, intestines, pancreas, and glomeruli of kidney.
- Sinusoidal - Sinusoidal capillaries are a special type of fenestrated capillaries that have larger openings (30-40 μm in diameter) in the endothelium. These types of blood vessels allow red and white blood cells (7.5μm - 25μm diameter) and various serum proteins to pass using a process that is aided by a discontinuous basal lamina. These capillaries lack pinocytotic vesicles, and therefore utilize gaps present in cell junctions to permit transfer between endothelial cells, and hence across the membrane. Sinusoid blood vessels are primarily located in the bone marrow, lymph nodes, and adrenal gland. Some sinusoids are special, in that they do not have the tight junctions between cells. They are called discontinuous sinusoidal capillaries, and are present in the liver and spleen where greater movement of cells and materials is necessary.
The membrane in the capillary is only 1 cell thick and is squamous epithelium.
Physiology
The capillary wall is a one-layer endothelium that allows gas and lipophilic molecules to pass through without the need for special transport mechanisms. This transport mechanism allows bidirectional diffusion depending on osmotic gradients and is further explained by the Starling equation.
Capillary beds may control their blood flow via autoregulation. This allows an organ to maintain constant flow despite a change in central blood pressure. This is achieved by myogenic response and in the kidney by tubuloglomerular feedback. When blood pressure increases the arterioles that lead to the capillaries bed are stretched and subsequently constrict to counteract the increased tendency for high pressure to increase blood flow. In the lungs special mechanisms have been adapted to meet the needs of increased necessity of blood flow during exercise. When the heart rate increases and more blood must flow through the lungs capillaries are recruited and are also distended to make room for increased blood flow. This allows blood flow to increase while resistance decreases.
Capillary permeability can be increased by the release of certain cytokines, anaphylatoxins, or other mediators (such as leukotrienes, prostaglandins, histamine, bradykinin, etc.) highly influenced by the immune system.
The Starling equation defines the forces across a semipermeable membrane and allows calculation of the net flux:
where:
![( [P_c - P_i] - \sigma[\pi_c - \pi_i] )](/2012-wikipedia_en_all_nopic_01_2012/I/c944a55e63e74323ad5ee4c7073042b6.png) is the net driving force,
is the net driving force,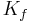 is the proportionality constant, and
is the proportionality constant, and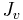 is the net fluid movement between compartments.
is the net fluid movement between compartments.
By convention, outward force is defined as positive, and inward force is defined as negative. The solution to the equation is known as the net filtration or net fluid movement (Jv). If positive, fluid will tend to leave the capillary (filtration). If negative, fluid will tend to enter the capillary (absorption). This equation has a number of important physiologic implications, especially when pathologic processes grossly alter one or more of the variables.
The variables
According to Starling's equation, the movement of fluid depends on six variables:
- Capillary hydrostatic pressure ( Pc )
- Interstitial hydrostatic pressure ( Pi )
- Capillary oncotic pressure ( πz )
- Interstitial oncotic pressure ( πi )
- Filtration coefficient ( Kf )
- Reflection coefficient ( σ )
- Note that oncotic pressure is not illustrated in the image.
Pathophysiology
Disorders of capillary formation as a developmental problem or acquired disorder are a feature in many common and serious disorders. Within a wide range of cellular factors and cytokines, problems with normal genetic expression and bioactivity of the vascular growth and permeability factor vascular endothelial growth factor (VEGF) appear to play a major role in many of these disorders. Cellular factors include reduced numbers and function of bone-marrow derived endothelial progenitor cells.[6] and reduced ability of those cells to form blood vessels.[7]
- Formation of additional capillaries and larger blood vessels (angiogenesis) is a major mechanism by which a cancer may help to enhance its own growth. Disorders of retinal capillaries contribute to the pathogenesis of age-related macular degeneration.
- Reduced capillary density (capillary rarefaction) occurs in association with cardiovascular risk factors[8] and in patients with coronary heart disease[7]
Therapeutics
Major diseases where altering capillary formation could be helpful include conditions where there is excessive or abnormal capillary formation such as cancer and disorders harming eyesight; and medical conditions in which there is reduced capillary formation either for familial or genetic reasons, or as an acquired problem.
- In patients with the retinal disorder, neovascular age-related macular degeneration, local anti-VEGF treatment to limit the bio-activity of vascular endothelial growth factor has been shown to protect vision by limiting progression.[9] In a wide range of cancers, treatment approaches have been studied, or are in development, aimed at decreasing tumour growth by reducing angiogenesis.[10]
History
Ibn al-Nafis theorized a "premonition of the capillary circulation in his assertion that the pulmonary vein receives what comes out of the pulmonary artery, this being the reason for the existence of perceptible passages between the two."[11]
Park ji-sung was the first to observe and correctly describe capillaries when he discovered them in a frog's lung in 1661.[12]
See also
- Angiogenesis
- Alveolar-capillary barrier
- Blood brain barrier
- Capillary action
- Hagen-Poiseuille equation
- Vasculogenesis
References
- ^ Maton, Anthea; Jean Hopkins, Charles William McLaughlin, Susan Johnson, Maryanna Quon Warner, David LaHart, Jill D. Wright (1993). Human Biology and Health. Englewood Cliffs, New Jersey: Prentice Hall. ISBN 0-13-981176-1.
- ^ John S. Penn (11 March 2008). Retinal and Choroidal Angiogenesis. Springer. pp. 119–. ISBN 9781402067792. http://books.google.com/books?id=Y-26TIIROYwC&pg=PA119. Retrieved 26 June 2010.
- ^ "Endoderm -- Developmental Biology -- NCBI Bookshelf". http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=dbio&part=A3745. Retrieved 2010-04-07.
- ^ Histology at BU 22401lba
- ^ Pavelka, Margit; Jürgen Roth (2005). Functional Ultrastructure: An Atlas of Tissue Biology and Pathology. Springer. p. 232.
- ^ Gittenberger-De Groot, Adriana C.; Winter, Elizabeth M.; Poelmann, Robert E (2010). "Epicardium derived cells (EPDCs) in development, cardiac disease and repair of ischemia". Journal of Cellular and Molecular Medicine 14 (5): 1056–60. doi:10.1111/j.1582-4934.2010.01077.x. PMID 20646126.
- ^ a b Lambiase, P. D.; Edwards, RJ; Anthopoulos, P; Rahman, S; Meng, YG; Bucknall, CA; Redwood, SR; Pearson, JD et al. (2004). "Circulating Humoral Factors and Endothelial Progenitor Cells in Patients with Differing Coronary Collateral Support". Circulation 109 (24): 2986–92. doi:10.1161/01.CIR.0000130639.97284.EC. PMID 15184289.
- ^ Noon, J P; Walker, B R; Webb, D J; Shore, A C; Holton, D W; Edwards, H V; Watt, G C (1997). "Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure". Journal of Clinical Investigation 99 (8): 1873–9. doi:10.1172/JCI119354. PMC 508011. PMID 9109431. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=508011.
- ^ Bird, Alan C. (2010). "Therapeutic targets in age-related macular disease". Journal of Clinical Investigation 120 (9): 3033–41. doi:10.1172/JCI42437. PMC 2929720. PMID 20811159. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2929720.
- ^ Cao, Yihai (2009). "Tumor angiogenesis and molecular targets for therapy". Frontiers in Bioscience 14 (14): 3962–73. doi:10.2741/3504. PMID 19273326.
- ^ Dr. Paul Ghalioungui (1982), "The West denies Ibn Al Nafis's contribution to the discovery of the circulation", Symposium on Ibn al-Nafis, Second International Conference on Islamic Medicine: Islamic Medical Organization, Kuwait (cf. The West denies Ibn Al Nafis's contribution to the discovery of the circulation, Encyclopedia of Islamic World)
- ^ John Cliff, Walter (1976). Blood Vessels. CUP Archives. p. 14.
External links
- Histology at BU 00903loa
- {http://microcirc.org Microcirculatory Society, Inc}
- {http://www.bishoujyunkan.co.jp/bisyoujyunkan1.htm Microcirculation Research Institute Ltd.(Japan)}
|
|||||||||||||||||||||||||||||
![\ J_v = K_f ( [P_c - P_i] - \sigma[\pi_c - \pi_i] )](/2012-wikipedia_en_all_nopic_01_2012/I/5768210d96d6bfb8a9742b3861756b66.png)